Description
ISO 16140-2 validated alternative method for the detection of Listeria monocytogenes and Listeria spp.
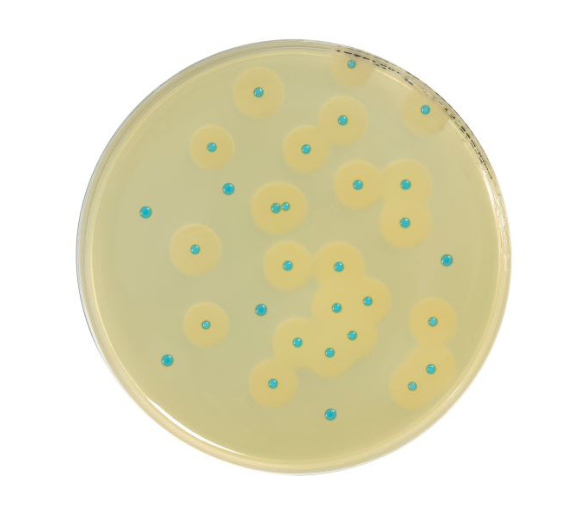
COMPASS® Listeria is a rapid alternative method used for the detection of Listeria monocytogenes and of Listeria
spp. in food products, and in environmental samples.
It features a single step of selective enrichment in Fraser ½ broth, followed by subculture onto COMPASS® Listeria
Agar. Enrichment can be performed at 37°C for 18 to 24 hours, or at 30°C for 22 to 28 hours.
This method is certified NF VALIDATION, according to the validation protocol NF EN ISO 16140-2 of 2016 for all
human food products and samples from the industrial production environment. The reference method used for the
validation is the standard NF EN ISO 11290-1 of 2017.
The method is certified NF VALIDATION, under Attestation N° BKR 23/02-11/02.
Please refer to the COMPASS LISTERIA AGAR_VALIDATED METHOD_BK192HA_BM12308_BM12408_BS07008_BS07108_BT00808
ISO 16140-2 validated alternative method for the enumeration of Listeria monocytogenes
The COMPASS® Listeria method is also used as a rapid alternative method for the enumeration of L. monocytogenes
in human food products and environmental samples, by surface or deep plating.
The method is certified NF VALIDATION, under Attestation N° BKR 23/05-12/07.
Please refer to the COMPASS LISTERIA AGAR_VALIDATED METHOD_BK192HA_BM12308_BM12408_BS07008_BS07108_BT00808
Normalized method for the detection and enumeration of Listeria monocytogenes et Listeria spp.
The formulation of the COMPASS® Listeria Agar corresponds to that recommended in the international standards NF
EN ISO 11290-1 and NF EN ISO 11290-2.
COMPASS® Listeria Agar is the first mandatory isolation medium in the L. monocytogenes and Listeria spp. detection
protocol, and the only medium in the L. monocytogenes and Listeria spp. enumeration protocol.
The formulation of the COMPASS® Listeria Agar also corresponds to Agar Listeria according to Ottaviani and Agosti in the Chapter 10 of FDA’s Bacteriological Analytical Manual (FDA-BAM).
Please refer to the COMPASS LISTERIA AGAR_acc. ISO 11290
Packaging
Ready-to-use medium:
BM12308 – 20 Petri plates Ø 90 mm
BM12408 – 120 Petri plates Ø 90 mm
Kit COMPASS Listeria Agar :
BT00808 – 6 vials of 200 mL base medium + 6 vials of freeze-dried selective supplement + 6 vials of liquid enrichment supplement
Dehydrated base medium:
BK192HA – 500 g bottle
Enrichment supplement:
BS07008 – 8 vials qsp 1L
Freeze-dried selective supplement :
BS07108 – 8 vials qsp 1L
Documentation
- FDS_MSDS_BK192HA_v1.3.pdf
- FDS_MSDS_BM12308_BM12408_v1.1.pdf
- FDS_MSDS_BS07108_v1.3.pdf
- FDS_MSDS_BT008_R1_v1.pdf
- FDS_MSDS_BT00808_R2_BS06708_v1.4.pdf
- Non classification statement_BS07008.pdf
- Non classification statement_BT00808_R3_BS06808.pdf
- TDS_COMPASS LISTERIA AGAR_acc. ISO 11290
- TDS_COMPASS LISTERIA AGAR_VALIDATED METHOD_BK192HA_BM12308_BM12408_BS07008_BS07108_BT00808